At its peak in April, New Jersey was seeing more than 4,000 COVID-19 cases per day. Hackensack Meridian Health, a large health system with 13 hospitals in the state, leveraged the research technologies in use at its John Theurer Cancer Center to analyze real-world evidence data on COVID-19 positive patients.
“As an oncologist, we already had an outcomes division. We already pull data out of the EHR and organize it in a way to track what’s doing well and what’s not doing well for cancer research. We had a system in place to do that with cancer, so why not shift our efforts and look to see how COVID patients are doing in hospitals, not just in our hospital but across the state,” says Stuart Goldberg, MD, a hematologist/oncologist and chief of the Division of Outcomes and Value Research at the John Theurer Cancer Center.
Using data from 3,000 hospitalized COVID-19 patients, Hackensack Meridian Health was able to determine that the famous malaria treatment, hydroxychloroquine, did not improve survival for hospitalized patients. Researchers were also able to determine that another drug, tocilizumab, actually improved survival among critically ill intensive care unit (ICU) patients.
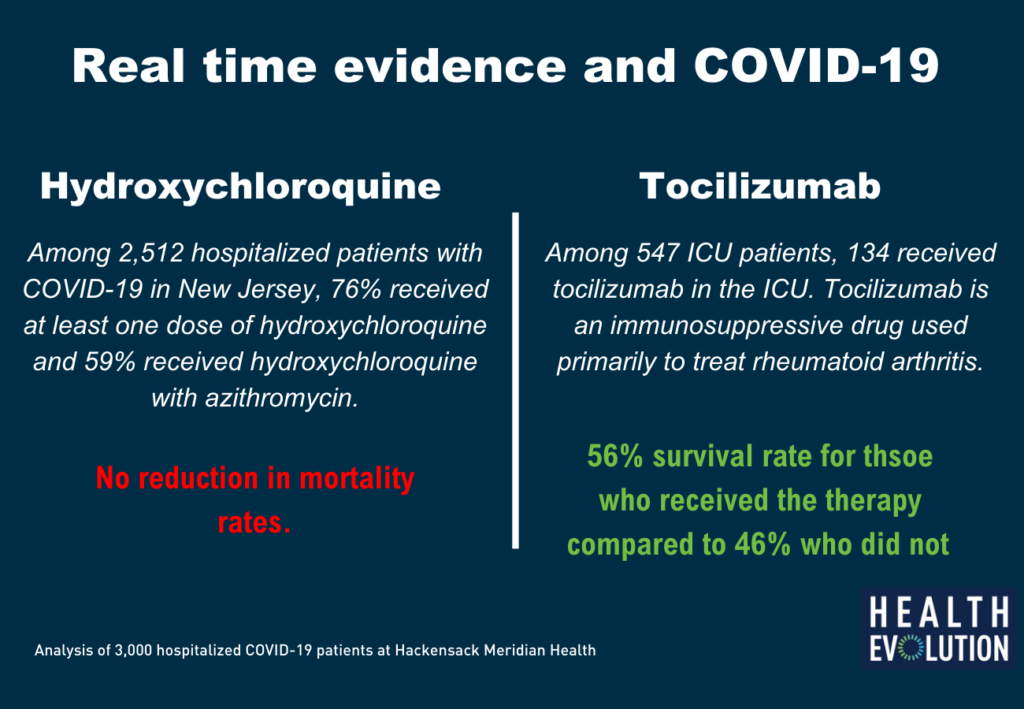
“We looked at the role of hydroxychloroquine in treating COVID-19 patients. It was a hot topic in the news, with President (Trump) touting the drug. It was a question people needed answered quickly,” Goldberg says.
The narrative on hydroxychloroquine has quickly changed as various private and public organizations have pulled the plug on trials to study its effectiveness.
Rise of real-world evidence
The rise of real-world evidence begun before COVID-19 ever entered the medical lexicon, but the virus has brought it to the forefront. Hackensack’s vendor partner, COTA, a tech company based in Boston, has joined the COVID-19 Evidence Accelerator program, a joint initiative of Friends of Cancer Research and the Reagan-Udall Foundation for the Food and Drug Administration.
“When we saw what was happening with humanity because of how ill-informed we were on COVID-19, and we still don’t understand the full biology of the virus, we decided we should redeploy our expertise and help,” says Mike Doyle, CEO of COTA. “We had an advantage because patients, life science companies and providers in oncology have been using real-world data for quite some time.”
Thanks to the 21st Century Cures Act, the Food and Drug Administration created a real-world evidence framework in December 2018. The FDA’s Amy Abernethy, MD, Principal Deputy Commissioner, who was featured on a recent Health Evolution Executive Briefing, has said that the pandemic will undoubtedly change the way real-world data sources are used going forward. Goldberg says the industry has yet to fully embrace the concept.
“I’ve been working with real-world data and these ideas for the last 6-7 years. In general, the studies using real-world data are not looked upon favorably by journals. Doctors looked at it as a secondary [data source],” Goldberg says.
However, he says COVID-19 will jump start real-world evidence by at least several years. “This whole data source can change the way we think about treating patients. Because of COVID and the need to treat patients fast and the need to quickly understand what’s working, it’s shifted our approach on real-world evidence.”
The rise of the EHR, he says, allows for health care organizations to mine data on what’s working—and to do it fast. While randomized clinical trials aren’t going anywhere, there isn’t enough time to wait years and years for the results and the development of a COVID vaccine, Goldberg adds. He says the biggest lesson from COVID should be health care’s willingness to use real-world evidence across the whole spectrum of the care system.
Challenges
For Hackensack Meridian Health, real-world evidence is possible because every hospital in its system is on the same Epic EHR system. However, if one of their patients got admitted to a hospital in New York City, in a facility that doesn’t use Epic, that outcome is lost from the system. Goldberg says health care’s lack of interoperability is one of the biggest challenges of real-world evidence.
Another challenge, Goldberg says, is finding good data scientists to mine the data quickly and effectively. Most data scientists in health care, he says, are used to looking at randomized clinical trials, whereas real-world data has biases that need to be corrected. “There are a lot of statistics that need to be applied to understand these associations. It’s a new way of thinking for doctors and scientists on what you can interpret from this and what is conjecture,” Goldberg says. “If you don’t apply good mathematics, you can very quickly give the wrong answer.”
The enormity of the COVID-19 pandemic has brought the health care industry together and moved it forward in ways not previously thought possible. This includes the research community and the way IT is used to accumulate evidence. Goldberg says health care CEOs and executives must pay attention to what’s happened with this pandemic and use technology to help clinicians make better decisions.
Read more: Health care executives put aside competitive differences to fight COVID-19
“If I’m a health care CEO or leader, you want to have the experience of every patient advanced to science. Up until now the computer has basically been a warehouse of data and hasn’t been utilized. If I’m a CEO, I want to change that not just because it’s good business, but because it will make our patients better and make your organization more efficient,” Goldberg says.











